How the EU regulatory context of the food contact materials and articles may change on our way to a toxic free environment − Over 44 years old EU rules under re-evaluation
The European Green Deal, Chemicals Strategy for Sustainability and Farm to Fork Strategy set the frame
European Union’s Chemicals Strategy for Sustainability (CSS) from October 2020 paves our path towards a toxic free environment. This is one of the goals, the European Green Deal growth strategy sets to tackle pollution and climate change. The ambition of the Green Deal is that the European Union (EU) will become a sustainable climate neutral and circular economy by 2050. As the CSS, also the European Commission’s Farm to Fork (F2F) Strategy from May 2020 is a part of the Green Deal aiming at a fair, healthy and environmentally friendly food system. Food Contact Materials (FCMs) are specifically mentioned in both strategies. The CSS focuses to protect the public health with the goal that the consumer products, including food contact materials, do not contain the most harmful chemicals. European Commission (EC) commits itself through the F2F Strategy to improve food safety and public health by revising the current FCM legislation. The main targets are at reducing the use of hazardous chemicals, supporting the use of innovative and sustainable packaging solutions of environmentally friendly, re-usable and recyclable materials, and contributing to food waste reduction. For example, single-use food packaging and cutlery should be replaced by re-usable products.
This blog intends to give you an overview of the main changes which the CSS and F2F Strategy may bring to the upcoming EU FCM regulations. Before giving the overview on the possible future FCM rules, the current EU regulatory framework of the FCMs and articles are described and discussed.
What are Food Contact Materials (FCMs) and articles?
Food Contact Materials (FCMs) are any materials which
i) are already in contact with food, such as milk cartons, yoghurt tubs, lemonade bottles, chocolate wrapping papers and sausage casings,
ii) are intended to be brought into contact with food, such as tableware, cooking utensils and food processing equipment, or
iii) can reasonably be brought into contact with food or transfer their constituents to the food under normal or foreseeable use, such as serviettes, paper napkins or tablecloths (case-by-case basis).
The FCMs and articles must be safe – both EU and national regulations apply
The FCMs should be sufficiently inert, so that their chemical constituents do not adversely affect consumer health and the quality of the food. The safety of the FCMs must be assessed because their constituents can migrate into food. In the EU, European Food Safety Authority (EFSA) evaluates the safety of the FCMs based on the applications submitted by the applicants. Following the favourable outcome of the EFSA assessment, the EC authorises the use of the FCMs. Only authorised FCMs are allowed to be used in the EU. However, in practise this concerns mainly plastics as EFSA does not normally evaluate non-plastic based FCMs. Nor the non-plastic based FCMs undergo the EU-authorisation process and the national statutory rules and procedures apply.
Currently the EU-wide harmonised FCM-legislation includes: The Framework FCM, Good Manufacturing Practise (GMP), some material specific (e.g. plastics, active and intelligent FCMs and ceramics), and some substance specific (e.g. bisphenol-A) regulations. For many different material types of the FCMs, such as paper, wood, printing inks, metals and glass, the national regulatory measures are applied, and no EU-wide harmonised legislation exists. The most commonly used of the latter ones are the German, French and Swiss national recommendations and regulations. Typically, national positive lists of the permitted chemical substances have been established by the different EU Member States (MSs). Recently, Germany updated its BfR-recommendations on FCMs and also indicated to set safety levels for mineral oil aromatic hydrocarbons (MOAH) in recycled food contact paper and cardboard. Where the national legislation applies, the principle on mutual recognition is used to bring the FCM or article approved by one EU MS for its national markets to the territories of the other EU MSs. Specifically in these situations, the National Food Safety Authorities can give useful advice on the national statutory requirements.
Nonetheless, whether the specific material-related legislation exists or not, the EU’s FCM legislation requires that any material and article must be manufactured in compliance with the good manufacturing practices (GMP, Regulation (EC) No 2023/2006) and the Framework FCM Regulation ((EC) No 1935/2004) in a manner that any potential transfer of a chemical constituent to food does not
i) endanger human health,
ii) change the composition of the food in an unacceptable way, or
iii) deteriorate the taste and/or odour of the food.
Furthermore, the EU-legislation stipulates that the labelling, advertising and presentation of a material or article shall not mislead the consumers. These two pieces of legislation cover all types of food contact materials and articles regardless of whether the FCM or article is directly or indirectly in contact with food.
Is my FCM active material?
Figure 1 depicts how the FCMs (substances and articles) are situated in the EU-regulatory context, and which regulatory actors are involved. Naturally, the FCMs and articles have a direct link to food regulations (e.g., food additives, food flavourings and food contaminants). But the FCMs and articles are also covered by other food regulations, such as official food control enforced by the EU MSs to control the traceability of the FCMs and their safety, or the recent transparency regulation when seeking for the EU-authorisations for the FCMs (Figure 1). Food additives and flavourings incorporated into the FCMs, and articles must not be released and cause technical effects in or on the food. If they do so, the FCM or article is considered as an active FCM or article because it is non-inert. Only food additives and food flavourings authorised in the EU can be used in the active FCMs and articles. The active contact materials should extend the shelf-life of the packaged food by maintaining or improving its condition, by releasing substances to or absorbing substances from the food or its surrounding environment.
FCMs under the REACH, CLP and BPR Regulations
In addition to the food law, the important EU chemicals regulations, REACH and CLP, apply to the FCMs. European Chemicals Agency (ECHA) is the body who implements the chemicals legislation in the EU (Figure 1). The REACH registration, restriction, and authorisation obligations as well as the CLP-provisions are also required to be followed for the substances and mixtures used to manufacture the FCMs. If the substances of very high concern (SVHCs) on the RECAH Candidate List are present at concentrations greater than 0.1% w/w, a SCIP-notification under Waste Framework Directive (WFD) applies for the food contact articles, too. Biocidal Products Regulation (BPR) can apply in cases where biocides are incorporated into the FCMs and articles (Figure 1). As other chemical constituents, the biocides must not be released from the FCMs and articles at levels negatively impacting the human health and the food quality.
Migration of the chemical FCM-constituents must be known
The safety of the FCMs and articles must be guaranteed by the business operator (in practice typically a manufacturer), and migration of the chemical FCM-constituents (also non-intentionally added substances, e.g., impurities and reaction products) to food has to be determined either by mathematical calculations, modelling or migration testing. Many research institutes and laboratories specialised to these tests, modelling and calculations provide commercial services to the FCM-business in Europe. Based on the generated results, they also issue certificates for the compliance of the material with the FCM-legislation in the EU and internationally. The certificates are requested by the customers of the FCMs as well as local food inspectors. Dietary risk assessment of the migrating substances from the FCMs and articles to food should also be conducted by the manufacturers where the guidelines, recommendations and regulations stipulating the safe levels are absent.
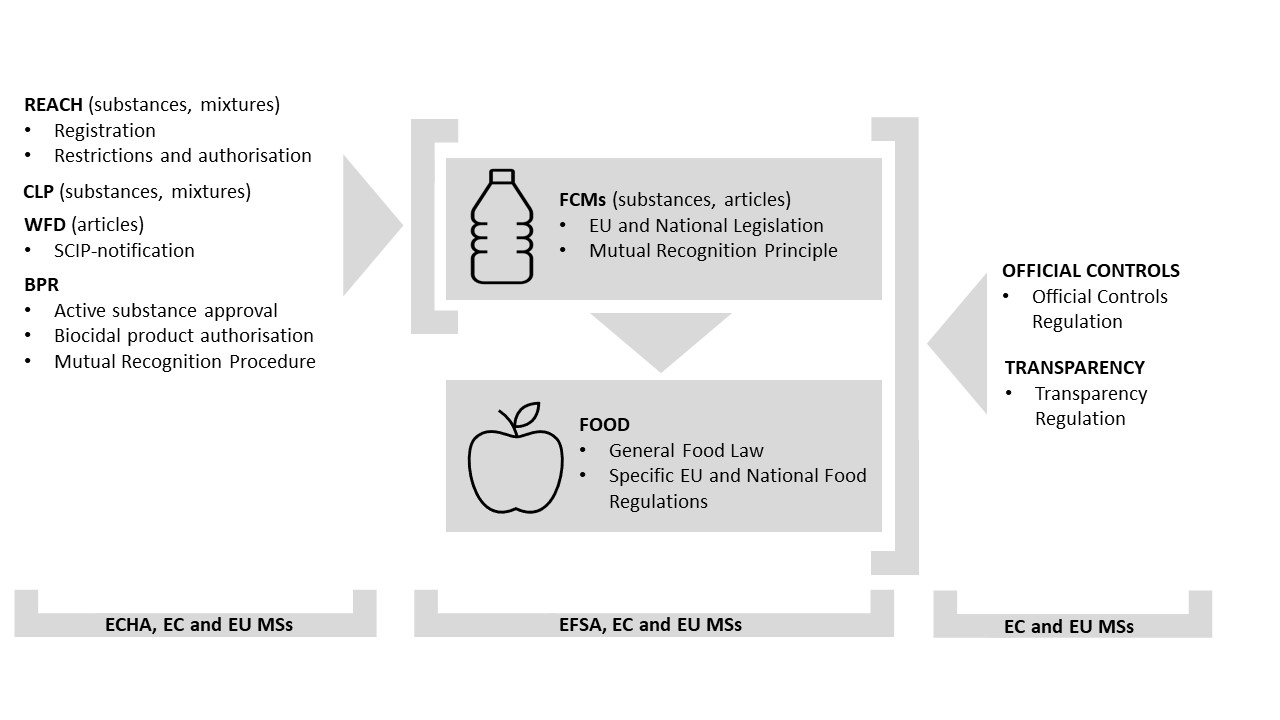
Figure 1: Food Contact Materials (FCMs) and articles in their regulatory context in the European Union.
Single-use plastics and other regulatory developments
It is also good to bear in mind the other regulatory developments in the EU and worldwide, such as single-use plastics, under which many food contact articles fall, and the packaging waste which also regulates food contact materials and articles. Recycling has an important role in both statutory acts. It is worth noting that the food contact materials and articles made partly or fully from the recycled plastics can only be from those recycling processes which have been authorised in the EU following the EFSA risk assessment. It is foreseen that the new the FCM-legislation will be extended to include the re-use of all types of FCMs and recycling.
From this July onwards, the EU MSs must ensure that certain single-use plastic products are no longer placed on the EU market. In May 2021, the EC published guidelines on the application of the single-use-plastic rules; for example, single-use biodegradable/ bio-based plastic products and paper-based products with plastic lining or coating fall under the single-use plastic definition. Overall, it is good to realise when considering the use of plastics, that also the global multinational food companies have large programmes to reduce the use of virgin plastics and enhancing their recycling. It is clear that the use of plastics will decline in the future, even though it is very vital packing material to keep our food safe in the increasingly complex global food and drink markets.
Ongoing reassessment of the EU FCM legislation – how it may change?
Since the establishment of the basic EU FCM legislation over 44 years ago, it has never been re-evaluated until now. This process started in 2017 and has revealed some concerns owing to the absence of the harmonised EU-level regulations for many other FCMs other than plastics. Several key problems have been identified, such as lack of focus on the final materials and articles while the current approach bases on positive lists, lack of prioritisation for the most hazardous substances and lack of encouragement to develop safer and sustainable alternatives. Since the inception of the re-evaluation, the strategies on the chemicals (i.e., CSS) and farm to fork (i.e. F2F) were published as noted in the introduction. The most relevant possible regulatory changes to take place for the FCMs and articles in the EU are summarised in Table 1. The Table also indicates whether this change is origin from the CSS.
A shift from specific substances to final materials
A shift from the focus on the safety of the chemical substances to the full characteristics of all final materials and articles is expected to be included in the revisited FCM-legislation. In this context the GMP rules will be strengthened, too. It is also considered to reduce the complexity of the packaging materials, including the number of materials and polymers used today.
The CSS outlines a banning of the most harmful chemicals in consumer products including FCMs
The CSS outlines that the most harmful chemicals are to be banned and thus phased out in consumer products for non-essential uses. The most harmful chemicals will only be allowed in the consumer products if their use is essential for the society and no alternatives are available. The CSS lists that the FCMs and articles are among the consumer products which will be ensured to be free from substances causing cancers or mutations, affecting reproductive and endocrine systems, or being persistent and bioaccumulative by applying generic risk management. The CSS further outlines that this approach could be extended to the chemicals exerting other types of adverse effects later-on. How the “essentiality for the society” regarding the use of harmful chemicals in the FCMs will be considered in the future is to be seen. The question arises though, are the FCMs and articles considered essential for the society?
All substances, including non-intentionally added substances, and mixtures that may pose a health risk to consumers are envisaged to be considered in the new FCM legislation. Tiered approach to prioritise the assessment of these substances will be used. The most harmful chemicals (carcinogenic, mutagenic and reprotoxic substances (CMRs), endocrine disruptors (EDs), persistent, bioaccumulative and toxic substances (PBT and vPvBs) are in the first tier, and the other specific substances (e.g., nano-forms) are in the second tier. The official EU risk assessment bodies ECHA and EFSA are to conduct the assessments for these substances, while the third-tier substances can be self-assessed by the business operator. The latter ones are the least of concern and those which migrate at low levels. A generic approach based on the hazardous properties (e.g., carcinogenicity) will be used to prioritise the substances, with certain defined exceptions. These exceptions may include the FCMs if considered essential.
Chemicals to be assessed as groups
The CSS also describes that grouping of chemicals with similar properties (hazard, structure, risk and/or function) will be used to assess the substances instead of regulating them one-by-one. This could mean for example that a group of chemicals with similar hazardous properties are banned or restricted in one go. This will impact also the chemicals used to manufacture the FCMs. The SCC emphasises the phase out of PFAS as a group, this includes their use also in the FCMs (unless essential for society). Some RECAH restriction intentions already exist for PFAS. Netherlands, Denmark, Germany, Norway, and Sweden are working on a proposal for a European-level ban of PFAS. Recently, at the national level Denmark banned the use of PFAS-substances in food contact paper and cardboard, and articles thereof. Bisphenol A and its structurally related analogues as well as phthalates could also be such groups to be restricted or banned in the near future.
The ban of endocrine disruptors in consumer products is specifically highlighted in the CSS and it is likely that none of them are allowed to be used in the FCMs in the future. The CSS details that criteria to identify endocrine disruptors need to be included also in the FCM legislation. The criteria have already been defined for the biocides and plant protection products regulations. The upcoming RECAH and CLP revisions both foresee the introduction of endocrine disruptors in them. Endocrine disrupting substances are of high concern also at the national level in Europe. For example, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) has identified 16 priority substances to be assessed urgently for their endocrine disrupting properties.
One-substance-one-assessment for FCMs jointly done by ECHA and EFSA
The CSS also sets out a principle of one-substance-one-assessment. For the FCMs this means that EFSA and ECHA will assess the FCM-substances and mixtures in a close cooperation. The FCMs are the first common regulatory area of EFSA and ECHA where one-substance-one assessment is applied. Interesting is to see how this will work out in practice as generally EFSA bases its assessments on risks, while ECHA bases its assessments on hazards. Could there be of a concern that restricting or banning of substances, or a group of substances based on hazards assessed by ECHA occurs far before than any FCM-application has been submitted to EFSA for the assessments of the dietary risks? It needs to be kept in mind that the REACH obligations for substances and mixtures are to be obeyed prior to the manufacturing of the FCMs can start and any FCM-application is submitted to EFSA. Therefore, it might be that the RECAH-level identification of the substances and mixtures of concern will become more influential in the future than the EFSA-level dietary risk assessment in the future. It could be anticipated that the EFSA risk assessments will continue only for those FCMs which have not been identified for a ban by ECHA or for those which have been derogated from the bans.
In addition to EFSA-ECHA joint work with the FCMs and REACH, they also cooperate with FCMs and biocides. In March 2021, they published a joint document where the two bodies identified several differences in their risk assessments for silver compounds used as biocidal active substances in the FCMs. Currently, EFSA, ECHA and EC are jointly defining the procedures and best practises for the one-substance-one assessments. Time will tell how this will function.
Mixtures of substances are of a high interest
The adverse effects from mixtures of chemical substances, so called “cocktail-effects”, are also to be considered within the EFSA risk assessments on the FCMs. In the recent past, EFSA has published several guidance documents and other information on the chemical mixtures. Many international developments are ongoing on the chemical mixtures. The mixture assessment factor is also to be introduced in the revised REACH for the safety assessment of the chemicals as outlined by the CSS. The chemical mixtures are currently a hot scientific and regulatory topic, and certainly FCMs are to be impacted by these developments.
Polymers to be registered under REACH
The CSS specifically draws attention to polymers, as today they are not subject to registration under the REACH. Therefore, in the updated REACH, the registration of polymers of toxicologically concern is foreseen. This will naturally, one way or other, have an influence on the plastic-FCMs. Furthermore, the REACH will be amended on information requirements irrespective of the manufactured or imported volume of the chemicals to enable the identification of critical hazardous properties also for the low and medium tonnages. Some other recent scientific developments have indicated that other types of polymer-materials, such as cellulose and bio-based plastics, manifest some toxicological effects. Whether the new FCM-plastic legislation is extended also to these chemicals is to be realised.
Declaration of Compliance (DoC) to be introduced for all types of FCMs
Clear and consistent regulatory rules for the data requirements as well as the rules for the information transfer through the supply chain will be set in the updated FCM-regulations. This is to improve the information flow. The requirement for the business operator to issue a Declaration of Compliance (DoC) will be introduced for all types of FCMs. Presently, coatings, printing inks and adhesives are not subject to the requirement of a DoC, except when used in plastic materials and articles. For the other FCMs, the DoC needs to be provided as dictated by the FCM Framework Regulation. A DoC structure is available in the current plastic regulation.
New business opportunities to develop safe and sustainable FCMs
The newly developed chemicals need to be safe and sustainable by design as presented by the CSS. Therefore, safer, and more sustainable alternatives are to be promoted, and the EC anticipates revising the FCM legislation including specific new rules on the safety of more sustainable production sources and methods. The EC will also provide incentives to produce safe and sustainable FCMs, e.g., using bio- and plant-based technologies. Thus, EU-funding will be available to research and develop new greener food contact materials. Furthermore, the FCM-legislation will be expanded to include the re-use of all types of FCMs and recycling. The FCM sustainability could include the aspects, such as the amount of energy used in the FCM-manufacturing and its renewability, extended self-life of the food due to the use of new FCM-types, reduced use of raw materials, re-use, and recycling of the FCM and the overall environmental footprint of the FCM. It is likely that these considerations will also be taken into account in the future assessments of the FCMs. Table 1 lists the main expected regulatory changes in the updated FCM and articles legislation.
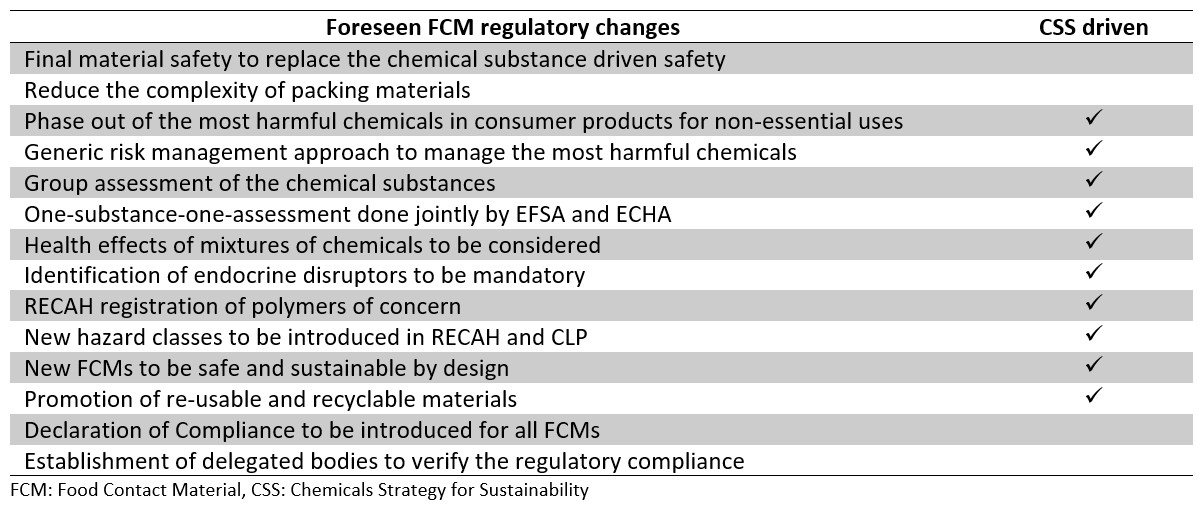
Table 1: The main possible regulatory changes to take place in the updated FCM and articles legislation in the European Union.
Two possible ways to move forwards in the new FCM-legislation
Two possible ways to move forwards in the new FCM-legislation have been proposed by the EC: either to revise the current regulatory framework (to have Regulation (EC) No 1935/2004 as a corner stone) or to develop a new regulatory framework, replacing the current regulation. Time will tell which option the EC and the EU MSs will choose. It is planned that the EC will adopt the new FCM-legislation by the end of 2022. However, delays may occur. New provisions are also anticipated to be added to the Official Food Control Regulation to establish new delegated bodies to carry out inspections and enforcement of the FCM rules. A system will be developed to verify the compliance with the FCM-legislation. It is yet to be seen how this will evolve.
Finally, I hope this blog shed some light on the upcoming revisions and adjustments in the EU’s FCM regulatory field. I wish you found some food for the thought and that this blog fed you to think about what may lie ahead. Below you find some further reading on the new regulatory developments and the references on which the text above bases.
As you just read, many changes will take place in the food contact materials and articles legislation in the coming years. Under the current European growth strategy Green Deal, many of these evolvements will be inevitable. Therefore, stay tuned, follow the Ecobio websites, use our Ecobio Manager to keep up with the regulatory changes and contact us for your open questions!

